Published in: R. Spiewak (Editor): "Pollens and Pollinosis: Current Problems". Institute of Agricultural Medicine, Lublin (Poland) 1995, pages 100-102. (The book's table of contents.)
The aim of the present work was to evaluate whether systemic application of specific allergen during the immunotherapy causes rhinomanometrically detectable functional changes in the nasal cavity. If such effect exists, rhinomanometry might be useful in the monitoring of the so-called subclinical side effects of the specific immunotherapy (SIT), which is very important for performing the effective but at the same time safe SIT. Because of relevant interindividual differences of sensitivity in patients, in every case the physician should choose the antigen dosis individually, basing on as broad as possible spectrum of clinical data [6]. The objective of this study was therefore to examine whether the rhinomanometry is an appropriate tool to detect the effects of subcutaneows antigen application on nasal patency in pollinotic patients with seasonal allergic rhinitis (SAR).
16 SAR patients, 7 females and 9 males, aged 7 to 43 years (see Table 1).
The Homoth rhinomanometer was used. This rhinomanometer is a part of a multi-functional diagnostic device "HNO Diagnostik Center" produced by Homoth, Germany. The study was performed outside the pollen season. After a 30-min acclimatisation to the examination room, in each patient five measurements in ten-minutes intervals were carried out. The nasal resistance during inspiration and expiration was measured at the differential pressure of 150 Pa. Immediately after the second measurement, the patients who were before qualified to the SIT, received an initial dose of pollen vaccine "Catalet - T" (Biomed, Krakow) in amount of 0.1 ml 25 PNU solution, i. e. 2.5 biological units. After 10, 20, and 30 minutes the patients underwent subsequent rhinomanometric measurements. Because of the small size of the group tested, the data obtained were tested statistically with the use of the small sample paired signs test [3].
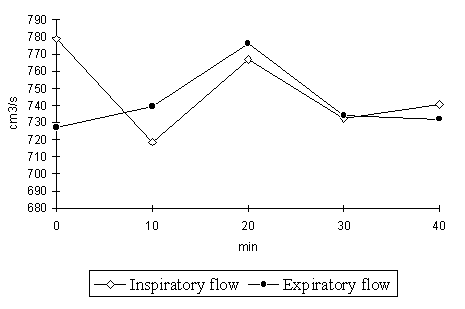
The results of systemic antigen administration in the group tested are shown Table 1. In the tested group the only statistically significant (p=0.05) difference was observed between the nasal inspiratory flow values at the start of the experiment (Median=779 cm3 x s-1) and after 30 minutes since the antigen administration (Median = 740.5 cm3 x s-1). Analysis of the graph (Fig. 1) suggests that because of a considerable variability of the parameters measured the conclusions should be drawn with caution. In none of the patients any side effects after receiving the immunotherapy were observed. None had complained of feeling any nasal blockage either.
Table 1. The influence of systemic antigen application on the nasal patency
|
Measurement (minute of observation) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 (0. min) |
2 (10. min) |
3 (20. min) |
4 (30. min) |
5 (40. min) |
||||||||||
No |
Sex |
Age |
Ins |
Exp |
Ins |
Exp |
Ins |
Exp |
Ins |
Exp |
Ins |
Exp |
||
1 |
M |
30 |
616 |
570 |
637 |
670 |
490 |
357 |
409 |
496 |
444 |
464 |
||
2 |
F |
43 |
615 |
638 |
553 |
723 |
417 |
789 |
333 |
763 |
630 |
858 |
||
3 |
F |
18 |
797 |
799 |
776 |
806 |
793 |
843 |
804 |
855 |
762 |
832 |
||
4 |
M |
18 |
805 |
742 |
648 |
788 |
658 |
812 |
701 |
682 |
746 |
485 |
||
5 |
F |
16 |
786 |
726 |
722 |
694 |
772 |
747 |
785 |
747 |
735 |
711 |
||
6 |
M |
33 |
934 |
908 |
884 |
897 |
762 |
718 |
585 |
548 |
859 |
805 |
||
7 |
M |
8 |
532 |
578 |
640 |
638 |
604 |
536 |
524 |
518 |
466 |
504 |
||
8 |
M |
7 |
772 |
728 |
812 |
756 |
835 |
800 |
768 |
721 |
643 |
590 |
||
9 |
M |
14 |
693 |
695 |
715 |
669 |
777 |
811 |
789 |
819 |
691 |
710 |
||
10 |
M |
26 |
791 |
834 |
805 |
897 |
786 |
763 |
604 |
700 |
771 |
842 |
||
11 |
F |
6 |
375 |
372 |
488 |
428 |
329 |
392 |
400 |
354 |
377 |
398 |
||
12 |
M |
12 |
656 |
659 |
668 |
589 |
692 |
735 |
764 |
850 |
758 |
753 |
||
13 |
F |
35 |
822 |
770 |
800 |
881 |
821 |
830 |
771 |
758 |
800 |
809 |
||
14 |
F |
17 |
843 |
871 |
785 |
919 |
828 |
871 |
784 |
823 |
748 |
815 |
||
15 |
F |
31 |
844 |
803 |
818 |
785 |
817 |
807 |
809 |
779 |
782 |
788 |
||
16 |
M |
25 |
595 |
606 |
594 |
501 |
524 |
575 |
644 |
537 |
593 |
513 |
||
Median |
779 |
727 |
718.5 |
739.5 |
767 |
776 |
732.5 |
734 |
740.5 |
732 |
||||
Minimum |
375 |
372 |
488 |
428 |
329 |
357 |
333 |
354 |
377 |
398 |
||||
Maximum |
934 |
908 |
884 |
919 |
835 |
871 |
809 |
855 |
859 |
858 |
||||
1, 2, 3, 4, 5 - results of subsequent measurements in 10-minutes intervals; Ins- total nasal flow during inspiration at differential pressure of 150 Pa, the value was computed by adding the right- and left-side nasal flow measured during the inspiration; Exp - total nasal flow during expiration at differential pressure of 150 Pa, the value was computed by adding the right- and left-side nasal flow measured during the expiration |
||||||||||||||
Figure 1. Median changes of the total inspiratory and expiratory flow values at the differential pressure of 150 Pa in patients receiving the SIT using "Catalet - T" desensitising vaccine

The presented study was performed in patients at the beginning of their immunotherapy. Therefore, it can be assumed that the patients reactions were typical for the "natural" hypersensitivity. Ghaem et al. [2] observed correlation between specific IgE concentration and the nasal resistance increase during the allergen exposure. As shown in Table 1, 30 minutes after systemic application of the allergen a slight decrease of nasal patency was noted in the observed group. These data suggest that after the allergen application the nasal patency changes are very small and should not cause a relevant - from the patient's point of view - decrease of nasal comfort. Topically the airborne allergens cause an allergic reaction in the nasal mucosa which results in a nasal patency decrease [5]. In the presented study the allergen is carried by blood to the target organ. In the literature, the possibility of causing the allergic rhinitis by food allergens in the same way is discussed [4]. In both cases antigens pass to the nasal mucosa through blood.
The described observations are based on examination of the small group. If, however, they were confirmed in a larger population, it would mean that even a minimal allergen dose (2-3 biological units) having no effects on what a patient feels (no skin reaction in the application site, no systemic reactions), causes a detectable reaction in the shock organ. Without further research it would be difficult to foresee, whether expected diminishing of this phenomenon during the immunotherapy could be a sign of successful desensitization [1] or rather a sign suggesting that a too small dose had been administered [6]. Besides the nasal patency measurement, also other monitoring methods should be used parallelly e.g. specific IgG4 level determination [6] or, far less expensive and easy to perform, skin tests.
Rhinomanometry may be helpful as a monitoring method during the specific immunotherapy.
For personal use only. © Radoslaw Spiewak.
Page created: 29 April 2004, last updated: 13 February 2006.
| Contact Dr. Spiewak | Back to list of abstract | Website's front page |